400 Dowman Dr.
Math and Science Center
Room: N246
Phone: 404-727-4930
Email: lfinzi@emory.edu
Genetic Switches
Classic examples of the regulated transcription of genes are loop-based genetic switches that determine developmental choices such as quiescence versus virulence in bacteriophages or organ identity in plants. Studying these switches, offers a unique opportunity to discover ubiquitous regulatory principles. Loop-based genetic switches are present in all kingdoms of life. At their basis is the interaction between proteins bound at distant sites. This interaction causes the formation of a DNA loop. The looped and unlooped DNA state correspond to the ON/OFF (or viceversa) position of the switch. Investigating the conformations, thermodynamics and kinetics of different repressor-mediated genetic switches, we were able to point out the elements that are essential to make most loop-based genetic switches robust and yet responsive to environmental cues. Some of these essential elements are: modular binding sites with differential affinity for cooperative and tunable protein interactions, non-specific binding (in addition to specific binding) for synergy and molecular memory, DNA supercoiling for added stability and switching trigger, and a protein loop closure that can withstand high torsional stress to maintain the looped switch state in the face of varying torsion on the DNA outside the loop. The Figure below shows the molecular interactions that govern the switch from lysogeny to lysis in bacteriophage 186.
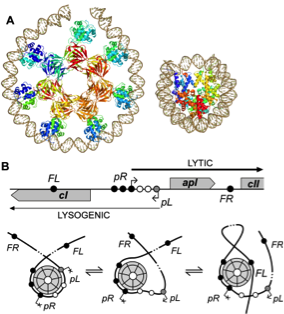
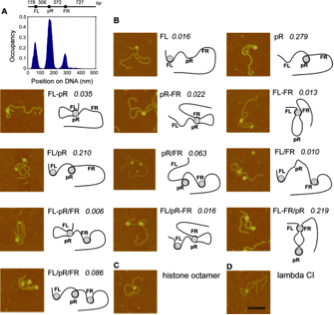
Figure adapted from H. Wang et al, NAR, 2013. Left: A - The 186 repressor is a heptamer of dimers which can wrap DNA. The nucleosome is shown besides it for scale comparison. B – (top) Schematic representation of the 186 DNA regulatory elements. pL is the lysogenic promoter from which the 186 repressor (CI) is transcribed; pR is the lytic promoter; solid black circles represent specific binding sites for 186 repressor DNA binding domains in the heptamer; empty circles represent non-specific binding sites. (bottom) Three possible 186 nucleoprotein conformations. Right: AFM analysis of the modes of interaction between 186 DNA and the 186 repressor protein. A – histogram showing the 186 Ci protein specific binding at the FL, pR and FR sites. B – AFM images showing the different nucleoprotein complexes detected and their frequency of occurrence. These different conformations play a role in the robustness of the 186 switch. C – histone octamer. D – lambda CI protein mediating a DNA loop.
Relevant Publications
| Authors | Title | Journal | Volume | Pages | Year |
| Yue Ding, Carlo Manzo, Geraldine Fulcrand, David Dunlap, Fenfei Leng and Laura Finzi | "DNA Supercoiling: a Regulatory Signal for the Lambda Repressor" | PNAS | 111(43) | 15402-15407 | 2014 |
| Marta Adelina Mendesa, Rosalinda Fiorella Guerra, Markus Christian Berns, Laura Finzi, Martin M. Kater and Lucia Colombo | “MADS-domain Transcription Factor Complex Induced Short-Range DNA Loop Formation is Essential for Target Gene Expression in Arabidopsis” | Plant Cell | 25 | 2560-2572 | 2013 |
| Haowei Wang, Ian Dodd, Keith Shearwin, David Dunlap and Laura Finzi | “Single molecule analysis of DNA wrapping and looping by a circular 14-mer of the 186 bacteriophage CI repressor” | Nucleic Acids Research | 41 | 5746-5756 | 2013 |
| Carlo Manzo, Chiara Zurla, David Dunlap and Laura Finzi | “The effect of non-specific binding of lambda repressor on DNA looping dynamics” | Biophys. J. | 103 | 1753-1761 | 2012 |
| Sachin Goyal, Chandler Fountain, David D. Dunlap, Fereydoon Family, Laura Finzi | “Stretching DNA to quantify non-specific binding” | PRE | 86 | 011905 | 2012 |
| Dale E.A. Lewis, Phuoc Le, Chiara Zurla, Laura Finzi, and Sankar Adhya | “Multi-level Auto-regulation of CI Protein in a λ lysogen” | PNAS | 108 (36) | 14807-14812 | 2011 |
| Paul Liebesny, Sachin Goyal, David Dunlap, Fereydoon Family and Laura Finzi | “Determination of the Number of Proteins Bound non-Specifically to DNA” | Journal of Physics: Condensed Matter | 22 | 414104 | 2010 |
| L. Finzi and D. Dunlap | “Single-molecule approaches to structure, kinetics and thermodynamics of transcriptional regulatory nucleoprotein complexes" | minireview - JBC | 285 | 18973-18978 | 2010 |
| C. Zurla, C. Manzo, D.D. Dunlap, D.E.A. Lewis, S. Adhya, L. Finzi | “Direct demonstration and quantification of long-range DNA looping by the λ bacteriophage repressor.” | NAR | 37 | 2789-2795 | 2009 |
| H. Wang, L. Finzi, D. Lewis and D. D. Dunlap | “AFM studies of the CI oligomers that secure DNA loops” | Curr. Pharmaceutical Biotechnology | 10 | 494-501 | 2009 |
| Suparna Sarkar-Banerjee, Sachin Goyal, Ning Gao, John Mack, Benito Thompson, David Dunlap, Krishnananda Chattopadhyay and Laura Finzi | “Specifically bound Lambda repressor dimers promote adjacent non-specific binding” | submitted |
Relevant Techniques
| Method | Used for |
| AFM | to image DNA-protein complexes and establish probabilities |
| TPM | to measure dynamics of loop formation |
| MTs | to measure how supercoiling affects genetic switches |
| FCS | to measure cooperative effects |