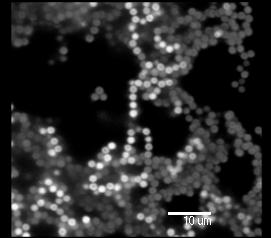
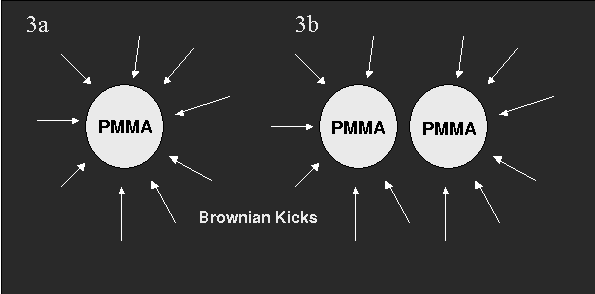
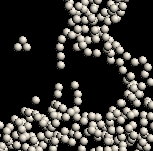With this project, we wish to investigate the behaviour of Colloidal Gels, an interesting example of a class of materials known simply as Gels - "a semi-solid colloidal system consisting of a solid dispersed in a liquid" [1].
In this context, semi-solid refers to the gels ability to hold its shape or resist (slight) deformation, despite its low solid concentration - as little as 0.1% solid for coagulated blood. Jell-O® or yogurt are good everyday examples of a gel.
Somehow then, in gels, a small amount of solid manages to support the weight of the whole system and thus maintain its shape. The solid-like character of these materials is invariably due to some sort of solid network that acts a little like a sponge, imprisoning the liquid within its structure. There exists a multitude of materials which can establish these networks with a wide range of structural complexity. In our case, the solid scaffolding's basic units are microscopic spherical particles called colloids; they form a gel because they are sticky (see below for details).
We have succeeded in making one such colloidal gel and we are currently studying the dependence of some of its properties on synthesis conditions as well as temperature and most interestingly, on its age.
What are we looking for?
We're just getting started, but we're curious about how the gel changes when we change the stickiness of the colloidal particles. We're also looking at the motion of the particles in the gel, again as we vary the stickiness. For a more detailed explanation of what we see, and why the particles are sticky, see below.What are we looking at? - The System
We use confocal microscopy to track fluorescently dyed PMMA (polymethylmethacrylate) particles in three dimensions. The particles are suspended in a density matched solvent so as to avoid sedimentation and floating.Also, we dissolved some polystyrene (PS) at various concentrations. Once in the solvent, the polystyrene collapses into a tight bundle with roughly spherical symmetry. We are thus effectively left with two types of particle in suspension: a large species and a small one. Preliminary measurements have set the size ratio of PMMA to PS of the order of 1:20 with the radius of the PMMA particles at 1.1E-6 m.