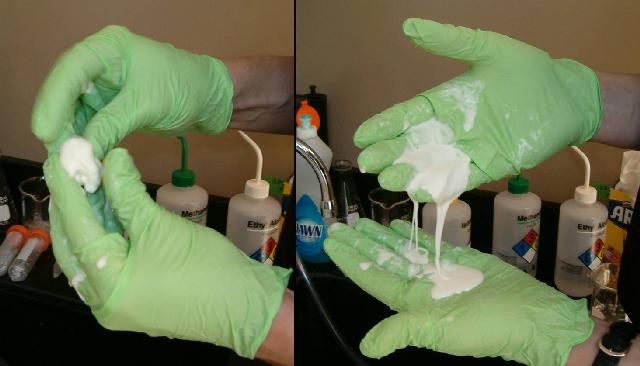
Left: When you quickly move corn starch mixed with water, the mixture acts like soft clay and can be rolled in your hands. Right: When allowed to flow slowly, the mixture flows like a liquid.
All of us wash our hair with shampoo, brush our teeth with a toothpaste, put various creams on our face, or spread some mayonnaise or ketchup on a sandwich. Few of us ever wonder what makes these substances behave the way they do. For instance, why do we have to shake out ketchup out of the bottle? Why doesn't it flow like water? Is it because it's more viscous than water, or does it depend on density? Is it really a liquid at all? Similar questions could be asked about tooth paste, shampoo, creams, corn starch, mud, paint, blood... These materials are called squishy materials - or more technically - complex fluids.
We have taught a 3-week unit about complex materials as a part of a freshman seminar class at Emory. For more information about this, you can download our preprint:
The original version contains more specific information about how we taught our course.

Left: When you quickly move corn starch mixed with water, the
mixture acts like soft clay and can be rolled in your hands. Right:
When allowed to flow slowly, the mixture flows like a liquid.
This work is supported by the NSF (Grant No. DMR-0239109). Any opinions, findings, and conclusions or recommendations expressed in this website are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.